Page 35 - Annual Report 2020
P. 35
response to intracellular pathogens; and (iii) Bax,
a pro-apoptotic protein that trans-locates to the mitochondria where it is activated to form pores in the MOM, leading to the release of cytochrome c and subsequently, apoptosis.
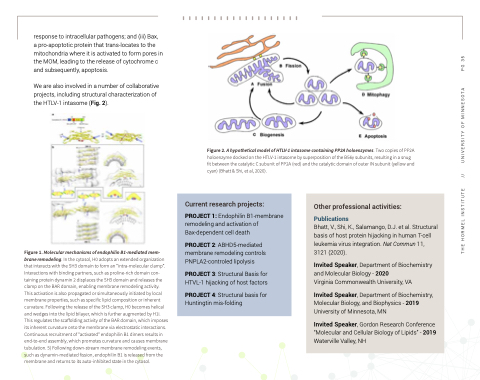
We are also involved in a number of collaborative projects, including structural characterization of the HTLV-1 intasome (Fig. 2).
Figure 1. Molecular mechanisms of endophilin B1-mediated mem- brane remodeling. In the cytosol, H0 adopts an extended organization that interacts with the SH3 domain to form an “intra-molecular clamp”. Interactions with binding partners, such as proline-rich domain con- taining protein dynamin 2 displaces the SH3 domain and releases the clamp on the BAR domain, enabling membrane remodeling activity. This activation is also propagated or simultaneously initiated by local membrane properties, such as specific lipid composition or inherent curvature. Following the release of the SH3 clamp, H0 becomes helical and wedges into the lipid bilayer, which is further augmented by H1i. This regulates the scaffolding activity of the BAR domain, which imposes its inherent curvature onto the membrane via electrostatic interactions. Continuous recruitment of “activated” endophilin B1 dimers results in end-to-end assembly, which promotes curvature and causes membrane tubulation. 5) Following down-stream membrane remodeling events, such as dynamin-mediated fission, endophilin B1 is released from the membrane and returns to its auto-inhibited state in the cytosol.
Figure 2. A hypothetical model of HTLV-1 intasome containing PP2A holoenzymes. Two copies of PP2A holoenzyme docked on the HTLV-1 intasome by superposition of the B56γ subunits, resulting in a snug fit between the catalytic C subunit of PP2A (red) and the catalytic domain of outer IN subunit (yellow and cyan) (Bhatt & Shi, et al, 2020).
Current research projects:
PROJECT 1: Endophilin B1-membrane remodeling and activation of Bax-dependent cell death
PROJECT 2: ABHD5-mediated membrane remodeling controls PNPLA2-controled lipolysis
PROJECT 3: Structural Basis for HTVL-1 hijacking of host factors
PROJECT 4: Structural basis for Huntingtin mis-folding
Other professional activities:
Publications
Bhatt, V., Shi, K., Salamango, D.J. et al. Structural basis of host protein hijacking in human T-cell leukemia virus integration. Nat Commun 11, 3121 (2020).
Invited Speaker, Department of Biochemistry and Molecular Biology - 2020
Virginia Commonwealth University, VA
Invited Speaker, Department of Biochemistry, Molecular Biology, and Biophysics - 2019 University of Minnesota, MN
Invited Speaker, Gordon Research Conference “Molecular and Cellular Biology of Lipids” - 2019 Waterville Valley, NH
THE HORMEL INSTITUTE // UNIVERSITY OF MINNESOTA PG 35