Page 7 - Annual Report 2020
P. 7
of melanoma or prostate cancer using standard needle vaccination procedures. As a result, local antigens presenting cells (APC) uploaded with the AAV-delivered tumor antigen either directly or by a cross-presentation pathway activate both a cytotoxic CD8+ T-cells and a humoral response against the tumor.
Our current research focused on the develop- ment and application of novel AAV vectors for purpose of gene therapy, ability of these vectors for immune system modulation, including both the induction and avoidance of antigen-specific responses depending on the therapeutic needs.
We are trying to develop the cancer AAV-based vaccine with a strong antigen-specific immune response after a single injection. We are designing novel viral system, which has overcome many
of the shortcomings of past viral cancer vaccine technologies: the precise modifications in the
AAV capsid and fuse of antigen with MHC class I molecule trafficking signals significantly increase transduction efficiency of the APCs and subse- quent antigen presentation. This modification significantly increase number of both antigen- specific CD8+ T and CD4+ T cells, enhanced the formation of effector memory precursor (CD 62L-/ CD127+) cells which ensure long-lasting anti-tu- mor immune response. Importantly, vaccination changes the immune landscape of the solid tumor by inducing massive invasion of immune cells, especially CD8+ T cells and NK cells. The use AAV based vaccination in combination with aPD-1 treat- ment significantly delayed tumor development, eliminate metastasis and extend in vivo survival.
We also are trying to develop approach to deter- mine the minimal effective vector dose to achieve a high level of expression with minimal immune response to the capsid. To this end, we engineered the capsids of AAV vectors to direct their cellular trafficking away from the phosphorylation-ubiq- uitination-proteasome degradation pathway and to improve transduction of human and murine hepatocytes. This approach of camouflaging AAV from cellular kinases can lead reduction of the immune response by capsid-specific CD8+ T-cell. We also demonstrated that transient suppression of the TLR9/MyD88/NF-kB pathway can minimize pro inflammatory response induced by AAV- mediated transduction of hepatocytes. We now are studying the cross-talk between GR and TLR9/ MyD88/NF-kB pathway during transduction of hepatocytes by optimized AAV vectors and a possible implementation for immune response
Non-vaccinated
Vaccinated
S669
S663
T665 K66
6
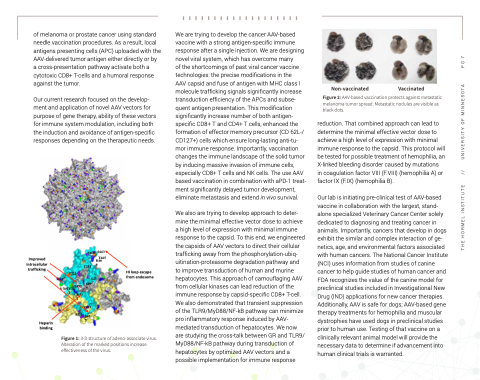
Figure 2: AAV-based vaccination protects against metastatic melanoma tumor spread. Metastatic nodules are visible as black dots.
reduction. That combined approach can lead to determine the minimal effective vector dose to achieve a high level of expression with minimal immune response to the capsid. This protocol will be tested for possible treatment of hemophilia, an X-linked bleeding disorder caused by mutations
in coagulation factor VIII (F.VIII) (hemophilia A) or factor IX (F.IX) (hemophilia B).
Our lab is initiating pre-clinical test of AAV-based vaccine in collaboration with the largest, stand- alone specialized Veterinary Cancer Center solely dedicated to diagnosing and treating cancer in animals. Importantly, cancers that develop in dogs exhibit the similar and complex interaction of ge- netics, age, and environmental factors associated with human cancers. The National Cancer Institute (NCI) uses information from studies of canine cancer to help guide studies of human cancer and FDA recognizes the value of the canine model for preclinical studies included in Investigational New Drug (IND) applications for new cancer therapies. Additionally, AAV is safe for dogs; AAV-based gene therapy treatments for hemophilia and muscular dystrophies have used dogs in preclinical studies prior to human use. Testing of that vaccine on a clinically relevant animal model will provide the necessary data to determine if advancement into human clinical trials is warranted.
Improved intracellular trafficking
K707
T251
T722
T492 Y73
HI loop escape from endosome
Y705 1
S47
2 S49
9
Y445 S551 S45
5
K53 1
Heparin binding
Figure 1: 3-D structure of adeno-associate virus. Alteration of the marked positions increase effectiveness of the virus.
THE HORMEL INSTITUTE // UNIVERSITY OF MINNESOTA PG 7